What is Potentiometric Titration ? Apa itu Titrasi Potensiometri ?
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
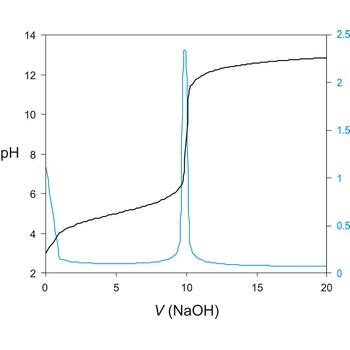
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
Potentiometric titration belongs to chemical methods of analysis in which the endpoint of the titration is monitored with an indicator electrode that records the change of the potential as a function of the amount (usually the volume) of the added titrant of exactly known concentration. Potentiometric titrations are especially versatile because indicator electrodes suitable for the study of almost every chemical reaction used in titrimetry are now available. This technique is also frequently used in the study of operational conditions of visual titrimetric indicators proposed for general use in chemical analysis, as well as in the study of numerous reactions, such as protonation and complexation, which find their application not particularly in analytical measurements. The course of the potentiometric titration curve provides information not only about the titration end point position, but also the position and shape of the curve may provide data about the processes accompanying the titration reaction. Another advantage is that the necessary apparatus is generally not expensive, reliable and readily available in the laboratories.
Source :
https://glossary.periodni.com/glossary.php?en=potentiometric+titration
https://www.sciencedirect.com/topics/chemistry/potentiometric-titration